TLR3 Knockout Immortalized Mouse Dendritic Cell Line
Cat. No.
T3034
Unit
1x10⁶ cells / 1.0 ml
Price
Inquiry
Worried about losing your cells due to growth or thawing difficulties, or even a random freezer breakdown? Enjoy peace of mind knowing that you can be covered under abm's Cell Line Insurance.
Sale of this item is subjected to the completion of a Material Transfer Agreement (MTA) by the purchasing institution.
For for-profit organizations, please contact quotes@abmgood.com for pricing.
Specifications
| Description |
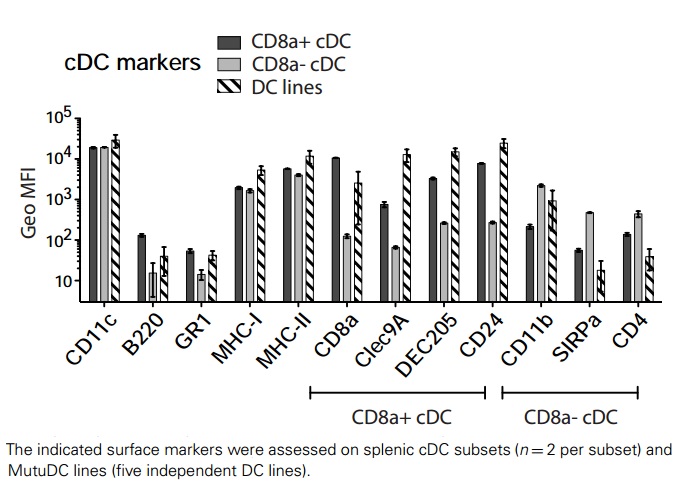
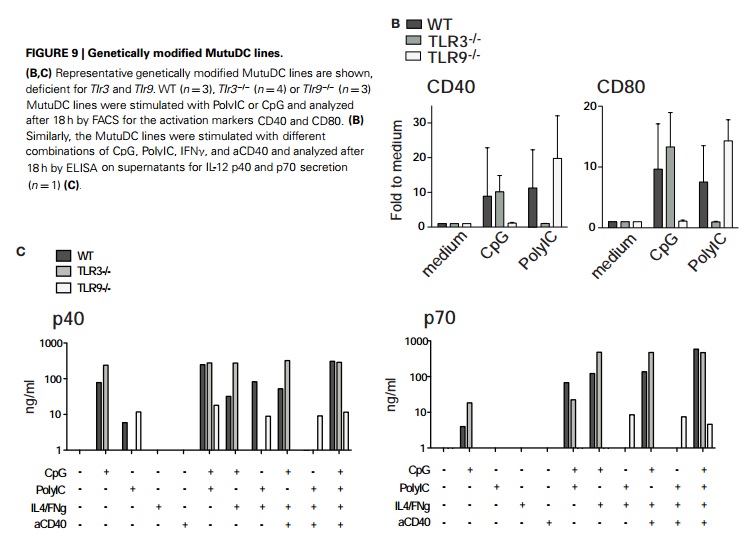
As messengers bridging the innate and adaptive immune system, the conventional tissue-resident DC (cDC) acts as sentinels in secondary lymphoid organs and other tissues for antigen capture and presentation. The MutuDCTlr3-/- cell line is an immortalized splenic CD8α subset (CD11chigh,B220-,DEC205 ,CD24high,CD11b-) of conventional dendritic cells derived from the TLR3 knockout CD11c:SV40LgT transgenic mice . The MutuDCTlr3-/- cell line retains response to TLR ligands such as CpG (TLR9-L) and to a lesser extent LPS (TLR4-L) but not PolyIC (TLR3-L). Like the Wild-type MutuDC cells (Cat. No. T0528), these cells are capable of presenting antigen in the context of both MHC-I and MHC-II, including direct antigen presentation and cross-presentation of cell-associated antigens. Together with TLR9 knockout (Cat. No. T3035) and Ifnar1 knockout (Cat. No. T3036) MutuDC, these dendritic cell lines provide a powerful tool in vaccine science and immunotherapy, particularly in strategizing target antigens to CD8α subset. |
| Cat. No. | T3034 |
| Name | TLR3 Knockout Immortalized Mouse Dendritic Cell Line |
| Unit | 1x10⁶ cells / 1.0 ml |
| Price | Inquiry |
| Category | Stable Cell Lines |
| Cell Type | Stable Cell Lines |
| Organism | Mouse (M. musculus) |
| Tissue | Spleen |
| Donor History | TLR3 knockout CD11c:SV40LgT transgenic mice |
| Cell Morphology | Small Aggregates |
| Growth Properties | Adherent, small aggregates |
| Seeding Density (cells/cm2) | 25,000 – 60,000 |
| Split Ratio | No greater than 1:3 |
| Expression Profile | CD11c, CD24, MHC-II+, DEC205, Clec9A, B220, CD11b, IRF4, IRF8, CD4 |
| Expression | CD11c, CD24, MHC-II+, DEC205, Clec9A, B220, CD11b, IRF4, IRF8, CD4 |
| Propagation Method |
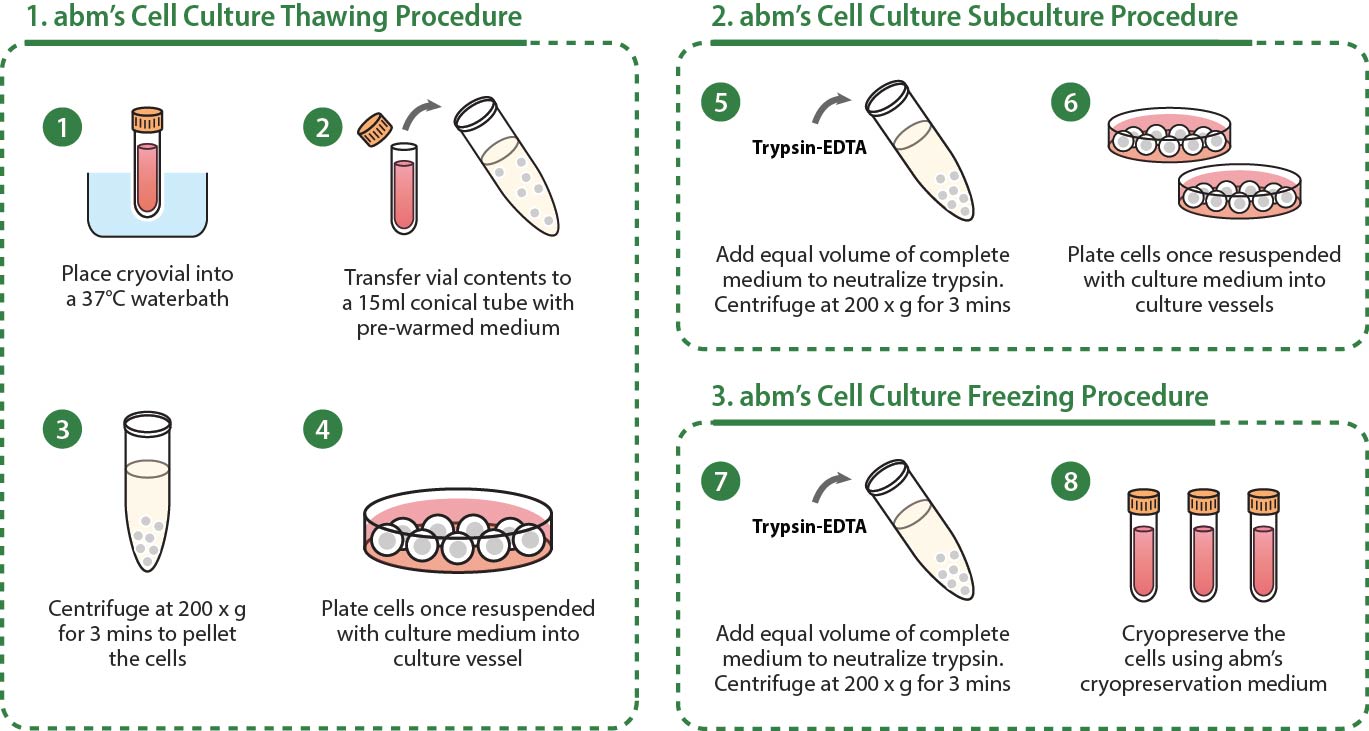
The base medium for this cell line is IMDM (1x) + GlutamaxTM (Gibco Ref: 31980-030). To make the complete growth medium, add the following components to the base medium: heat-inactivated fetal bovine serum (TM999) to a final concentration of 10%, 1% of 7.5% Sodium Bicarbonate Solution, 50 µM β-mercaptoethanol, HEPES to a final concentration of 10 mM, and Penicillin/Streptomycin Solution (G255) to a final concentration of 1%. Filter the complete media at 0.22µm before use. Carbon dioxide (CO2): 5%, Temperature: 37.0°C. To decomplement FBS: 1. Let the FBS bottle completely thaw overnight at 4°C. 2. Leave the FBS bottle in water bath for 30 minutes at 56°C. 3. Decomplemented FBS can be stored at -20°C for long term. Avoid frequent freeze-thaw cycling. Change the complete media every 2-3 days. Do not let media colour change to yellow. To thaw T3034: 1. Thaw the vial in 37oC water bath until there is no more than a small cube of ice. 2. Transfer the cells to a 15ml tube containing 5ml of pre-warmed complete media. 3. Centrifuge the cells at 290xg for 5 minutes. 4. Carefully discard supernatant without disturbing the cell pellet and gently resuspend the cells in 1ml of complete media by lightly pipetting up and down. 5. Seed the cells at 20,000 - 60,000 cells/cm2. To subculture T3034: 1. It is recommended to subculture when cells are at 70-90% confluency. 2. Aspirate old media. Some cells in suspension are still viable cells. Alternatively, the supernatant containing the suspended cells can also be collected and centrifuged (Skip to Step 6 in protocol). 3. Add 1:1 ratio of 1X sterile PBS and 0.25% Trypsin-EDTA (TM051). 4. Incubate cells at room temperature for 3-5 minutes and agitate the culture vessel until 90% of the cells have detached. 5. Immediately neutralize the trypsin by adding complete media equal to the volume of trypsin + PBS added. 6. Collect the cells and centrifuge at 290xg for 5 minutes. 7. Discard the supernatant and gently resuspend the cells in complete media by lightly pipetting up and down. 8. Recommended split ratio is no more than 1:3. Stimulation can be performed using PolyIC (5 µg/ml), CpG (2 mM) or LPS (5 µg/ml). These cells are especially sensitive to FBS requirement, thus, it is advised to use the same batch for culturing cells that show best result in supporting their culture. We also recommend the addition of extra 1% Hepes to the complete media to encourage propagation of the cells. To freeze T3034: Recommended freezing medium: Complete growth medium with decomplemented FBS to a final concentration of 50% and 5% DMSO. Storage temperature: Liquid nitrogen vapour phase. |
| Growth Conditions |
Use of PriCoat™ T25 Flasks (G299) or Applied Cell Extracellular Matrix (G422) is recommended for cell adhesion to the culture vessels. Prigrow V (TM015) + 4 mM Glutamax + 10% heat-inactivated FBS + 1% of 7.5% Sodium Bicarbonate Solution + 50 µM beta-mercaptoethanol + 10 mM HEPES (TM058) + 1% Penicillin/Streptomycin Solution (G255), 37.0°C, 5% CO₂. Filter the complete media with a 0.22µm filter prior to use. Change the complete media every 2-3 days. Do not let media colour change to yellow. |
| Subculture Protocol |
Volumes given below are for a T75 flask; proportionally increase or decrease the volume as required per culture vessel size. Subculture cells once the culture vessel is 80% confluent. Use a non-enzymatic, 5 mM EDTA-based cell dissociation buffer (5 mM EDTA in 20 mM Hepes-PBS) to subculture cells. 1. Aspirate excess media, and add 2-3 ml of the pre-warmed disssociation buffer mixture to the culture vessel, 2. Observe the cells under a microscope to confirm detachment (typically within 2-10 minutes). Cells that are difficult to detach can be put in 37°C, for several minutes to facilitate detachment. 3. Neutralize the solution by adding an equal volume of the complete growth media into the culture vessel. 4. Transfer the culture suspension into a sterile centrifuge tube, and centrifuge at 125xg for 5 minutes. The actual centrifuge duration and speed may vary depending on the cell type. 5. Aspirate the supernatant, and re-suspend the pellet with pre-warmed fresh complete growth media. Add appropriate aliquots of the cell suspension to new culture vessels, as desired. Cells must be seeded at high density. 6. Incubate the cells at the recommended conditions. |
| QC | 1) Direct antigen presentation and cross-antigen presentation were evaluated by the MHC-I (SIINFEKL/OT-I ) and MHC-II (OVA323-339/OT-II) restricted systems; 3) proteome profile and surface markers assessed by RT-PCR; RT-PCR; 3) IL-12 cytokine secretion analyzed using ELISA ; 4) Response to PAMP stimulation evaluated by functional assays. |
| Application | Research Use Only. |
| Storage Condition | Vapor phase of liquid nitrogen, or below -130°C. |
| BioSafety | II |
| Caution | For Research Use Only |
| Disclaimer |
1. Sale of this item is subjected to the completion of a Material Transfer Agreement (MTA) by the purchasing individual/institution for each cell line. If you have any questions regarding this, please contact us at licensing@abmgood.com. 2. All test parameters provided in the CoA are conducted using abm's standardized culture system and The stated values may vary under the end-user's culture conditions. Please verify that the product is suitable for your studies by referencing published papers or ordering RNA (0.5 μg, Cat.# C207, $450.00) or cell lysate (100 μg, Cat.# C206, $600.00) to perform preliminary experiments, or alternatively use our Gene Expression Assay Service (Cat# C138). All sales are final. 3. We recommend live cell shipments for ease of cell transfer and this option can be requested at the time of order placement. Please note that the end-user will need to evaluate the feasibility of live cell shipment by taking into account the final destination's temperature variation and its geographical location. 4. All of abm's cell biology products are for research use ONLY and NOT for therapeutic/diagnostic applications. abm is not liable for any repercussions arising from the use of its cell biology product(s) in therapeutic/diagnostic or any other non-RUO application(s). 5. abm makes no warranties or representations as to the accuracy of the information on this site. Citations from literature are provided for informational purposes only. abm does not warrant that such information has been shown to be accurate. 6. abm warrants that cell lines shall be viable upon initiation of culture for a period of thirty (30) days after shipment and that they shall meet the specifications on the applicable abm Material Product Information sheet, certificate of analysis, and/or catalog description. Such thirty (30) day period is referred to herein as the "Warranty Period". |
| Depositor | University of Lausanne (PACTT) |
Documents
Supporting Protocol
STR
There are no STR for this product yet!
FAQs
| I want to make sure these cells express my gene of interest before I decide to buy the cell line. Can you provide a sample so this can be tested? | |
|
We do not perform extensive downstream characterization, or gene expression profiling of our cell lines. However, you can check the ‘References’ tab to see if a publication is available, or you can contact us to see if RNA or cell lysates are available for your cell line of interest. Please directly contact us for further information at quotes@abmgood.com.
|
| Does abm's PriGrow series medium contain antibiotics? | |
|
No, the medium does not contain antibiotics and needs to be supplemented by the end-user as desired.
|
| Do I need to use abm's media and Applied Cell Extracellular Matrix (G422) to culture my cells? | |
|
We strongly encourage using the recommended media and culture conditions described in the "Growth Conditions" section as they have been optimized for cell growth. Other supplier's media and coating conditions have not been tested.
|
| What is the difference between Applied Cell Extracellular Matrix (G422) and Collagen Coating? | |
|
The main component of our Applied Cell Extracellular Matrix (G422) is type I collagen specifically. For more information on abm's Applied Cell Extracellular Matrix please visit: http://www.abmgood.com/Applied-Cell-Extracellular-Matrix-G422.html.
|
| Do I have to use T25 ECM-coated flasks for growing the cells? | |
|
We strongly recommend thawing cells in the T25 flasks specified on the cell-specific product page to ensure optimal post-thaw recovery of the frozen cells before testing other culture vessels.
|
| If I want to plate these cells to multi-well plates (e.g. 96 well plates) or dishes, how should I prepare the plates? | |
| What percentage of Trypsin-EDTA should be used to subculture cells? Can I use Trypsin-EDTA containing phenol red? | |
|
The standard concentration used for subculturing is 0.25% Trypsin-EDTA, available at abm (Cat. No. TM050). Yes, Trypsin-EDTA containing phenol red can be used as it does not interfere with trypsinization.
|
| How often do I need to change the media? | |
|
Media should be changed every 2-3 days or as specified within the recommended growth conditions.
|
| Why are these cells classified as biosafety level II? | |
|
We follow the CDC-NIH recommendations that all mammalian sourced products should be handled at the Biological Safety Level 2 to minimize exposure of potentially infectious products. This information can be found in 'Biosafety in Microbiological and Biomedical Laboratories' (1999). Your institution's Safety Officer or Technical Services will be able to make the call as to whether BioSafety Level I is possible with these cells at your site, if desired.
|
| How long can I store frozen vials? | |
|
Cells that are properly frozen using an effective cryoprotective agent can be stored in liquid nitrogen vapor phase (or below -130°C) indefinitely without affecting viability.
|
| What is the recommended storage temperature for cells? | |
|
In general, if you received:
|
| Can I substitute heat-inactivated FBS with FBS or vice versa? | |
|
FBS and heat-inactivated FBS are different in their composition; they cannot be substituted for one another.
|
| My cells are not detaching, what method do you recommend to trypsinize the cells? | |
|
| How are live cells shipped? | |
|
Live cells are shipped as a live culture in a T12.5 flask. Cells are seeded at an optimal confluency to avoid metabolic waste build up during transport. In addition, we thoroughly test our cell lines for functional freeze-thaw recovery. For more information on how to handle live cells, please view our detailed instructions here (https://www.abmgood.com/uploads/document/Cell_Handling_Instructions_Upon_Arrival_150623.pdf).
|
| Why are cells not attaching and forming clumps after subculturing with trypsin? | |
|
Cells form clumps and display attachment issues when the trypsinization agent is not effectively neutralized. To completely neutralize the trypsin agent, add an equal amount of complete growth media prior to centrifugation. Subtle changes in culture conditions, particularly in pH and the quality of serum used in the growth medium, may also affect the amount of clumping exhibited by the cells.
|
| Why are my cells forming clumps after plating? | |
|
It is important to ensure cell clumps are broken up through gentle re-suspension by pipetting up and down several times after pelleting cells by centrifugation. This will ensure a homogenous single cell suspension for even plating.
|
| Do I need to add the selection drug to the complete growth medium? | |
|
We recommend maintaining selection pressure using the drug concentration specified in the Growth Conditions section.
|
| Where can I find the CoA for my product? | |
|
Certificates of analysis can be found here (https://www.abmgood.com/CoA-Library.html).
|
References
- Fuertes Marraco, SA et al. "Novel murine dendritic cell lines: a powerful auxiliary tool for dendritic cell research" Front Immunol 3:331 (2012). DOI: 10.3389/fimmu.2012.00331.
Controls and Related Product:
Other Cell Lines
Select Species:
African Green Monkey (C. aethiops)
(0)
Bat
(0)
Bat (Chiroptera)
(0)
Bee
(0)
Bottlenose Dolphin (Tursiops)
(0)
Cabbage Looper (T. ni)
(0)
Cat (Feline)
(0)
Cattle
(0)
Chicken (Galline)
(0)
Chinese Hamster (C. griseus)
(0)
Cow (Bovine)
(0)
Deer (Cervidae)
(0)
Dog (Canine)
(0)
Duck (Anas)
(0)
Duck (Anatidae)
(0)
E. coli
(0)
European Sea Bass
(0)
European Sea Bass (D. labrax)
(0)
Fall Armyworm (S. frugiperda)
(0)
Fish
(0)
Fly (D. melanogaster)
(0)
Frog (Xenopus)
(0)
Fruit Fly (D. melanogaster)
(0)
Gilthead Sea Bream (S. aurata)
(0)
Goat (Capra)
(0)
Golden Hamster (M. auratus)
(0)
Goose (Anatidae)
(0)
Green Monkey (C. aethiops)
(0)
Green Monkey (C. sabaeus)
(0)
Hamster (M. auratus)
(0)
Horse (Equine)
(0)
Human
(0)
Human (H. sapiens)
(0)
Insect
(0)
Insect (Insecta)
(0)
Killifish (F. heteroclitus)
(0)
Luciola italica
(0)
Marmoset (Primate)
(0)
Mink
(0)
Mink (N. vison)
(0)
Monkey (Primate)
(0)
Moth (S. frugiperda)
(0)
Mouse
(0)
Mouse (M. musculus)
(0)
N/A
(0)
Other
(0)
Photinus pyralis
(0)
Pig (Porcine)
(0)
Quail (Coturnix)
(0)
Rabbit (Leporidae)
(0)
Rabbit (Leporine)
(0)
Rainbow Trout (O. mykiss)
(0)
Rat (R. norvegicus)
(0)
Rat (Rattus)
(0)
Renilla reniformis
(0)
Roundworm (C. elegans)
(0)
Rous Sarcoma Virus (RSV)
(0)
SARS-CoV-2
(0)
Sheep (Ovine)
(0)
Sheep (Ovis)
(0)
Turtle
(0)
Turtle (Testudines)
(0)
Universal
(0)
Zebrafish (D. rerio)
(0)
Select Tissue:
0
(0)
Achilles Tendon
(0)
Adipose
(0)
Adrenal Gland
(0)
Adrenal Glands
(0)
Airway
(0)
Airway/Lung
(0)
Amniotic Sac
(0)
Antimesometrial Decidua
(0)
Antler
(0)
Ascites
(0)
Auditory
(0)
Bee Cell
(0)
Bile Duct
(0)
Bladder
(0)
Blood
(0)
Blood (Immune)
(0)
Blood Vessel
(0)
Bone
(0)
Bone Marrow
(0)
Brain
(0)
Breast
(0)
CAF, Tumor
(0)
Caput Epididymis
(0)
Cartilage
(0)
Cerebrospinal Fluid
(0)
Cervix
(0)
Colon
(0)
Connective Tissue
(0)
Cord Blood
(0)
Digestive
(0)
Digestive System
(0)
Ear
(0)
Embryo
(0)
embryonic
(0)
Endometria
(0)
Endometrium
(0)
Esophagus
(0)
Eye
(0)
Fallopian Tube
(0)
Fibroblast
(0)
Gingiva
(0)
Hair
(0)
Hair Follicle
(0)
Head/Neck
(0)
Heart
(0)
HeLa
(0)
Hepatocellular Carcinoma
(0)
Hippocampus
(0)
Intestinal
(0)
Intestine
(0)
Kidney
(0)
Kidney, Cortex/Proximal tubule
(0)
Labial Salivary Gland
(0)
Larynx
(0)
Liver
(0)
Lung
(0)
Lung Carcinoma
(0)
Lung, bronchus
(0)
Lymph
(0)
Lymph node
(0)
Lymphatic System
(0)
lymphoblast
(0)
Malignant melanoma
(0)
Mammary
(0)
Mesencephalic Tissue
(0)
Mesentery
(0)
Microglia
(0)
Mouth/Oral
(0)
Muscle
(0)
Myoblast
(0)
Nasal Mucosa
(0)
Nerve
(0)
Nervous System
(0)
Oesophageal
(0)
Oesophagus
(0)
Oral
(0)
Organ of Corti
(0)
Other
(0)
Ovary
(0)
Pancreas
(0)
Pancreatic
(0)
Parathyroid
(0)
Penile
(0)
Peripheral Blood
(0)
Peripheral Blood Mononuclear Cells
(0)
Peritoneal
(0)
Peritoneum
(0)
Pharynx
(0)
Pituitary
(0)
Pituitary Gland
(0)
Pituitary Tumor
(0)
Placenta
(0)
promyelocytic leukemia
(0)
Prostate
(0)
Prostate carcinoma
(0)
Renal
(0)
Reproductive
(0)
Reproductive System
(0)
Retina
(0)
Salivary Gland
(0)
Sciatic Nerve
(0)
Sertoli
(0)
Skeletal
(0)
Skeletal Muscle
(0)
Skin
(0)
Skin ; Keratinocytes
(0)
Small intestine
(0)
Smooth Muscle
(0)
Soft Tissue
(0)
Spinal
(0)
Spleen
(0)
Stem Cell
(0)
Stomach
(0)
Straitum
(0)
Sweat Gland
(0)
Tendon
(0)
Testes
(0)
Testis
(0)
Thymic
(0)
Thymus
(0)
Thyroid
(0)
Tongue
(0)
Tonsil
(0)
Trachea
(0)
Tumor
(0)
Umbilical Cord
(0)
Unknown
(0)
Urethra
(0)
Urinary Tract
(0)
Uterus
(0)
Uterus; endometrium
(0)
Vascular Endothelium
(0)
Ventral Mesencephalon
(0)
Vertebral Disc
(0)